Congratulate! Zhenxing second generation Btk inhibitor approved for clinical application
2020-09-03
Zhenxing pharmaceutical obtained the approval notice of clinical trial following the "SM-1" project of the major science and technology special project of "national major new drug creation" in September 2019
Following the "SM-1" project of "national major new drug creation" in September 2019, Zhenxing pharmaceutical obtained the clinical trial approval notice. Another new drug zxbt-1158 capsule (B2 project) developed by Dongguan Zhenxing Beite Pharmaceutical Technology Co., Ltd., a holding subsidiary of our company, obtained the clinical trial approval notice from the food and drug administration on August 31, 2020. The product is intended to be used in the treatment of advanced relapsed and refractory B-cell malignant tumors.
Zxbt-1158 is a second-generation Btk inhibitor with high activity, which is based on the structure of acalabrutinib, an inhibitor of Bruton's tyrosine kinase (Btk). It has better selectivity and safety than the first-generation Btk inhibitor.
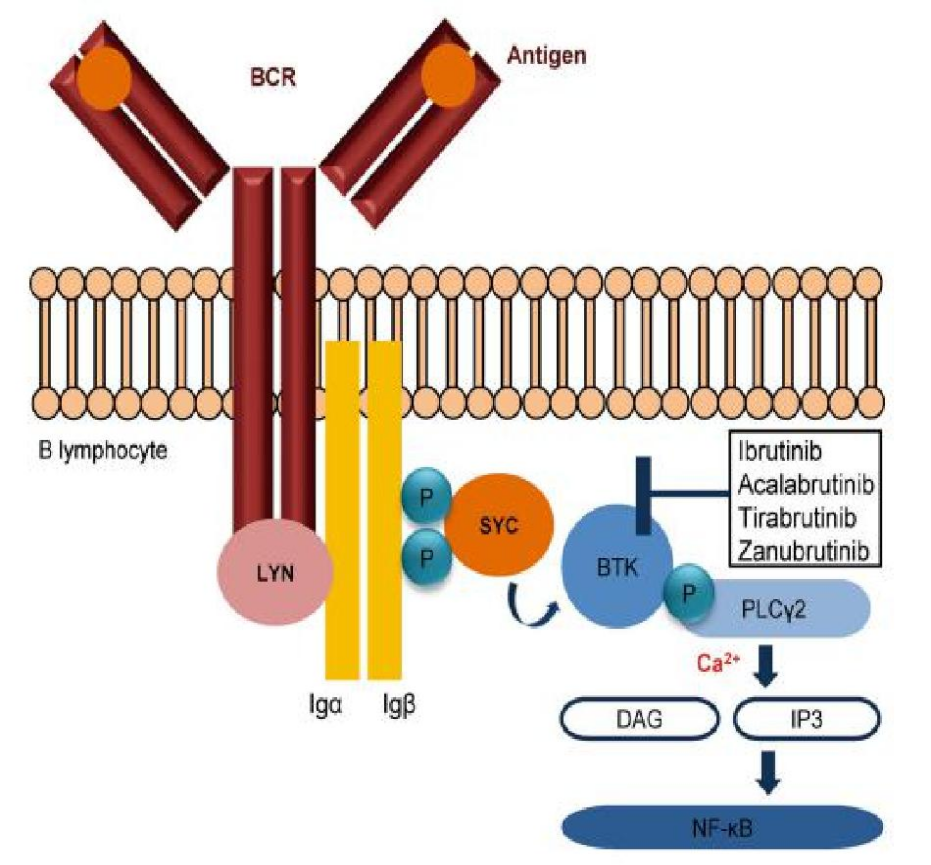
Studies have shown that zxbt-1158 can effectively inhibit the tumor growth of tmd-8 lymphoma model, and show superior drug characteristics. In addition to the treatment of hematolymphatic tumors, it may also be used in the treatment of rheumatoid arthritis and some solid tumors.
The approval of zxbt-1158 for clinical research is another important milestone in the development of Zhenxing medicine. I believe Zhenxing pharmaceutical will create greater market value in the future. At the same time, I would like to thank all sectors of society, shareholders and partners for their help to Zhenxing development.


